
Resources
Search for high-quality Active Pharmaceutical Ingredients through our extensive portfolio.
White Paper
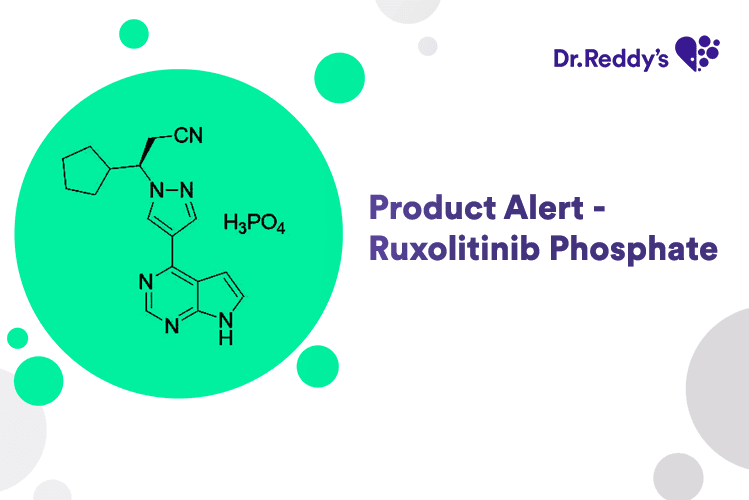
Jun 16, 2025
New Product Alert – Ruxolitinib Phosphate
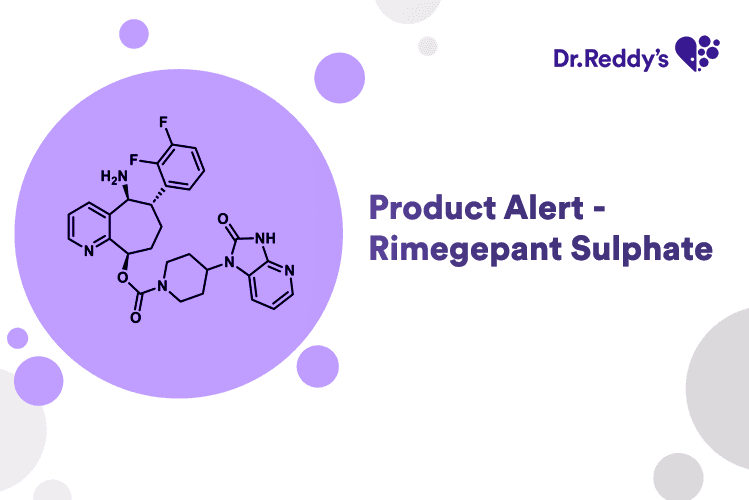
May 26, 2025
Product Alert - Rimegepant
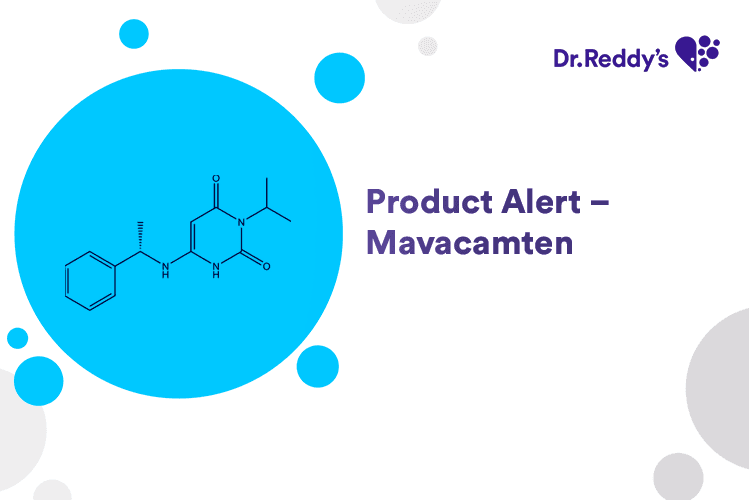
May 5, 2025
Product Alert – Mavacamten
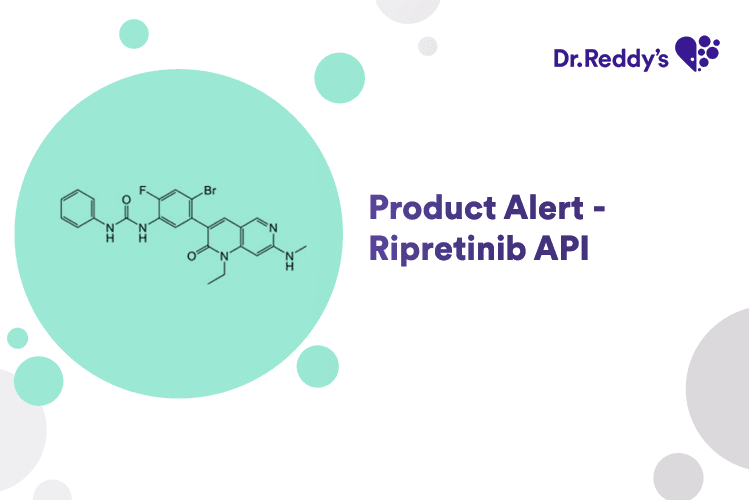
Apr 24, 2025
New Product Alert - Ripretinib
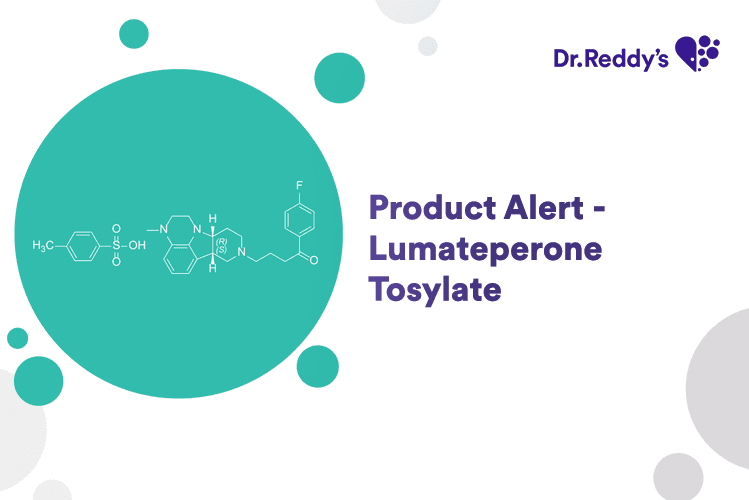
Mar 4, 2025
Product Alert - Lumateperone Tosylate
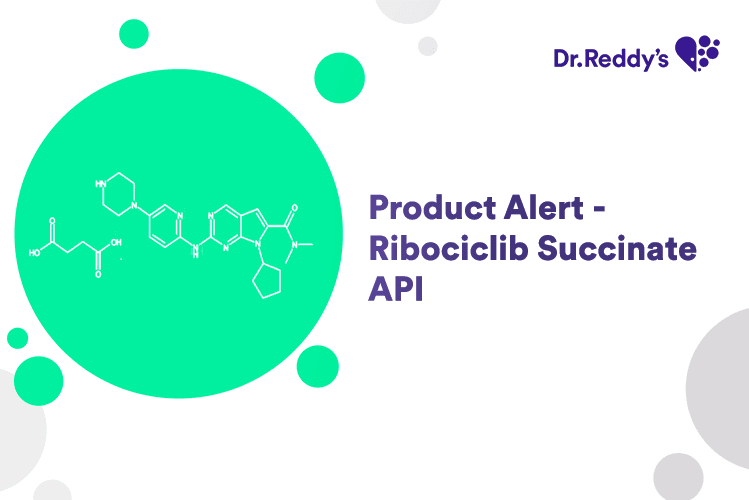
Feb 12, 2025
New Product Alert - Ribociclib
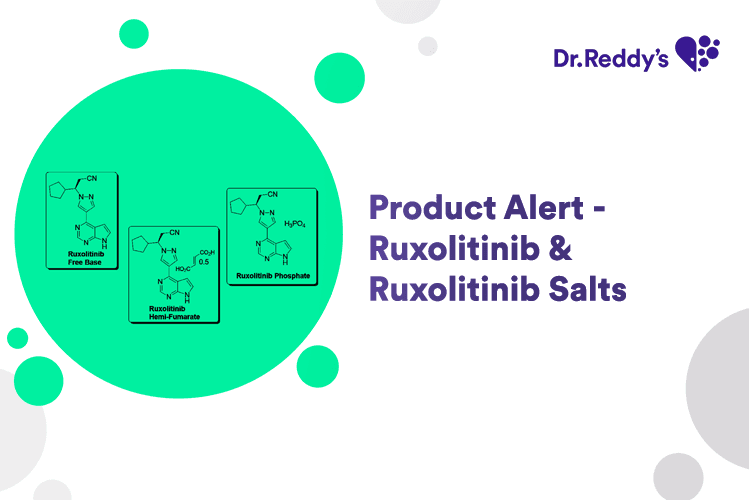
Jan 10, 2025
New Product Alert - Ruxolitinib & Salts
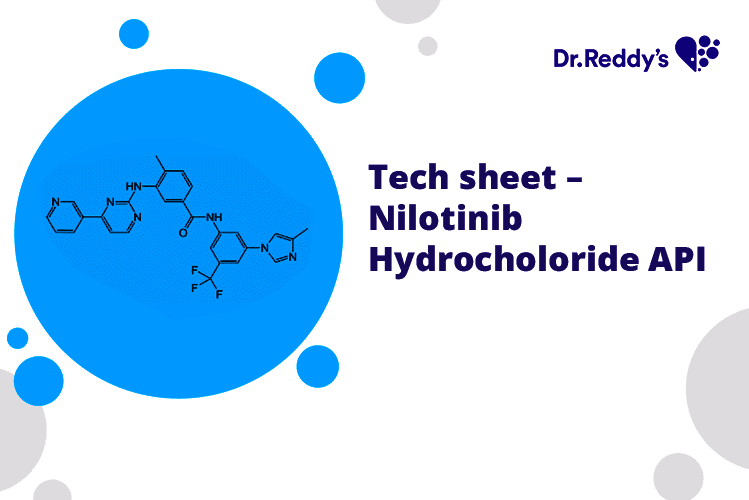
Dec 18, 2024
Tech Sheet On Nilotinib Hydrochloride
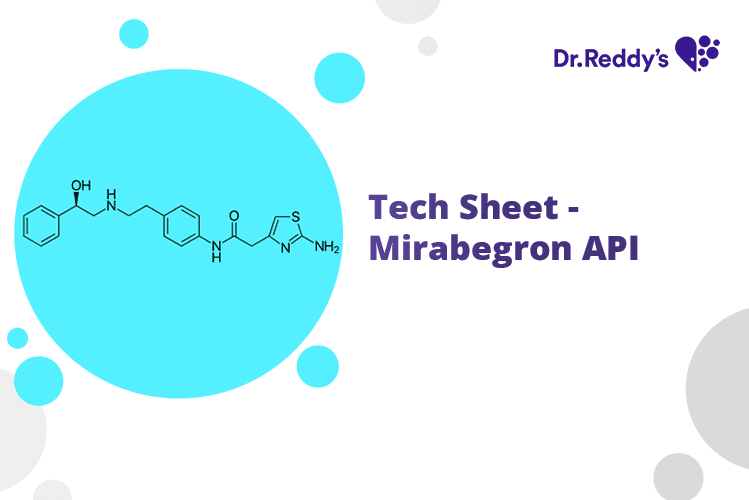
Oct 18, 2024
Tech Sheet – Mirabegron API
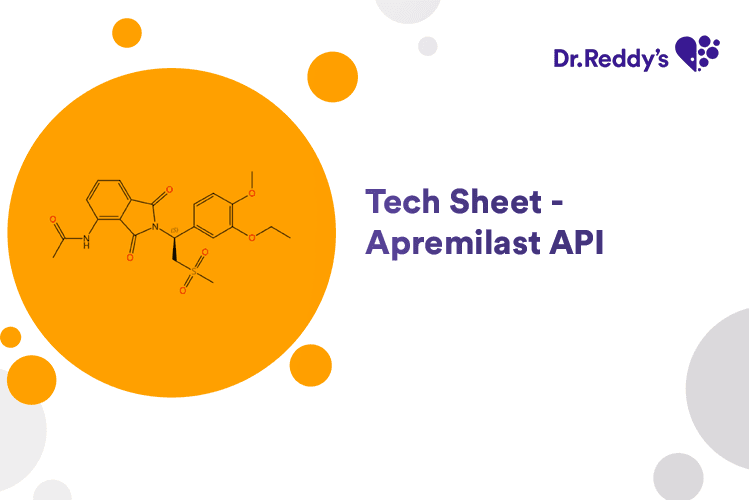
Sep 25, 2024
Tech sheet – Apremilast

Aug 4, 2024
Addressing the presence of mutagenic Azido impurities in Sartan APIs
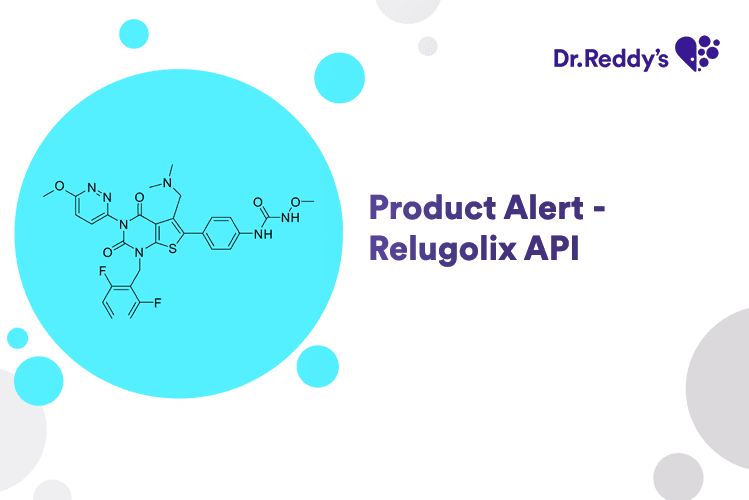
May 31, 2024
Product Alert - Relugolix API
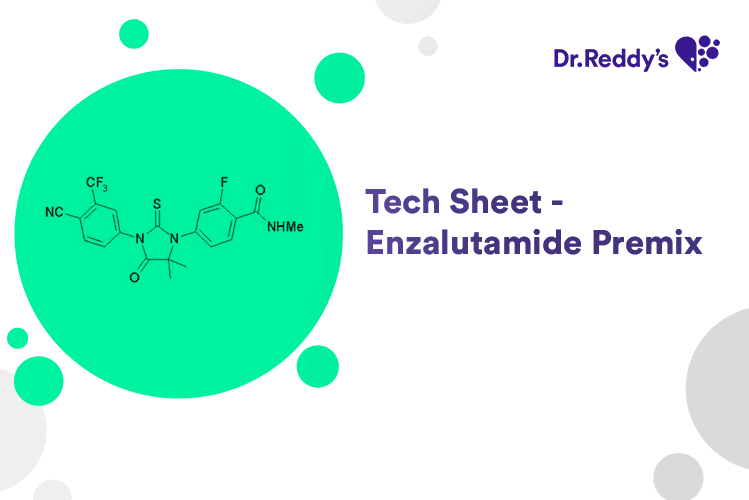
May 31, 2024
Tech Sheet on Enzalutamide Premix
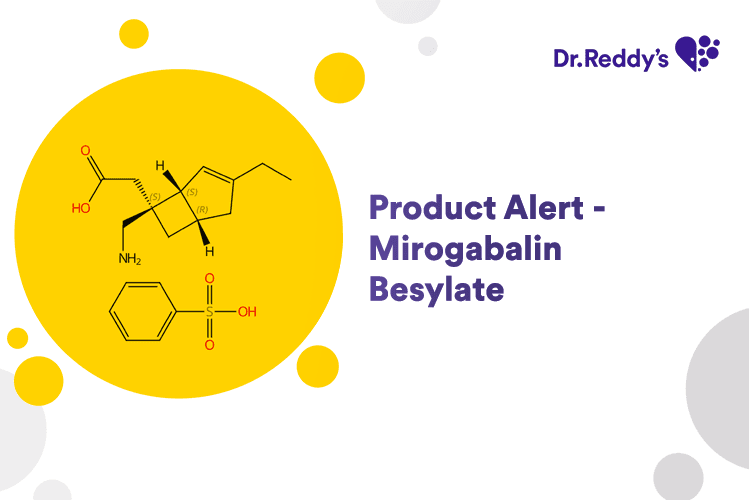
May 31, 2024
Product Alert – Mirogabalin Besylate
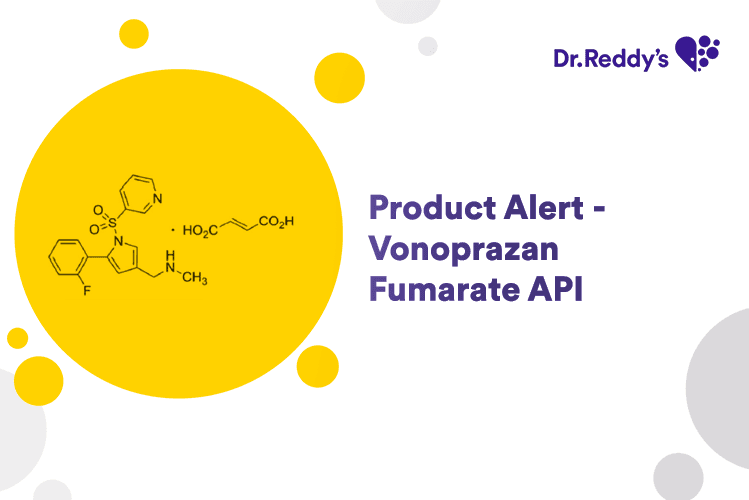
Apr 22, 2024
Product Alert – Vonoprazan Fumarate
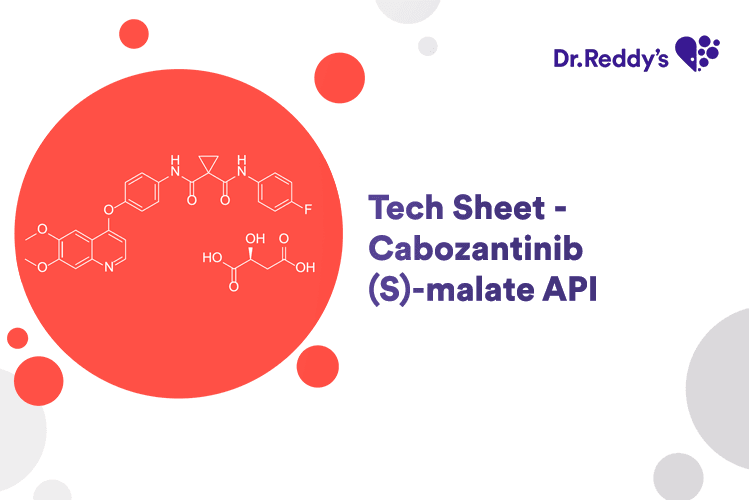
Feb 21, 2024
Tech Sheet - Cabozantinib (S)-malate API
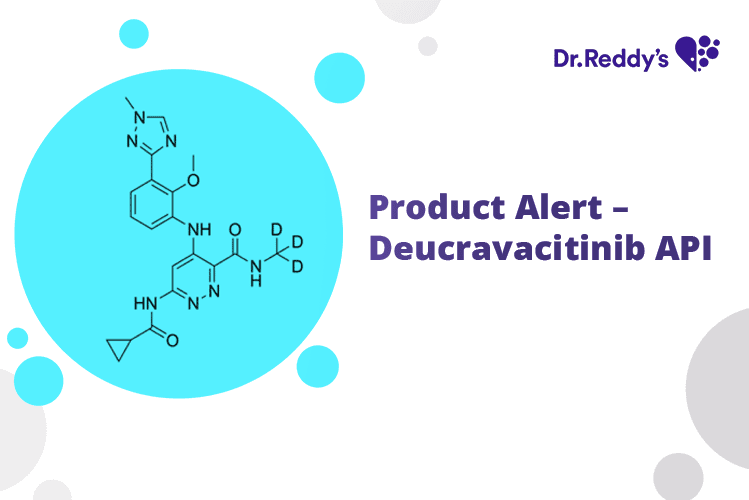
Dec 7, 2023
Product Alert – Deucravacitinib API
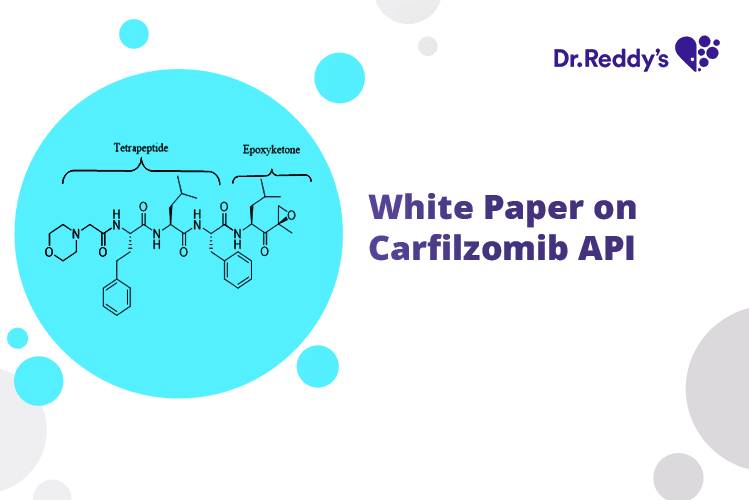
Dec 4, 2023
Substantially Pure Carfilzomib Amorphous for Generic Launch
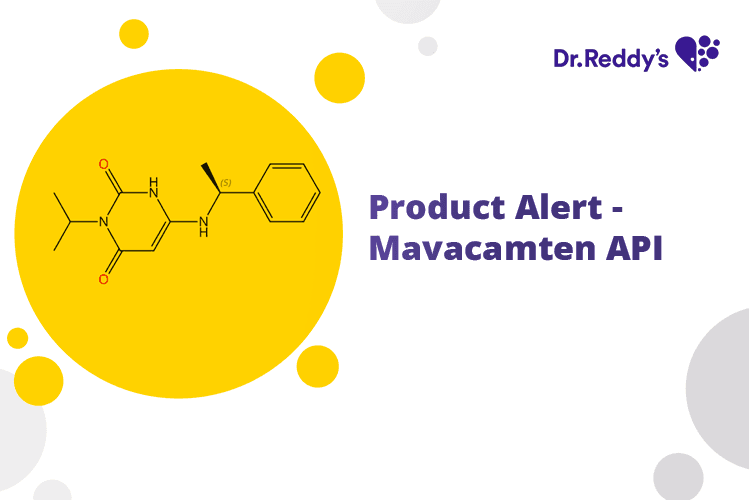
Nov 6, 2023
Product Alert – Mavacamten API
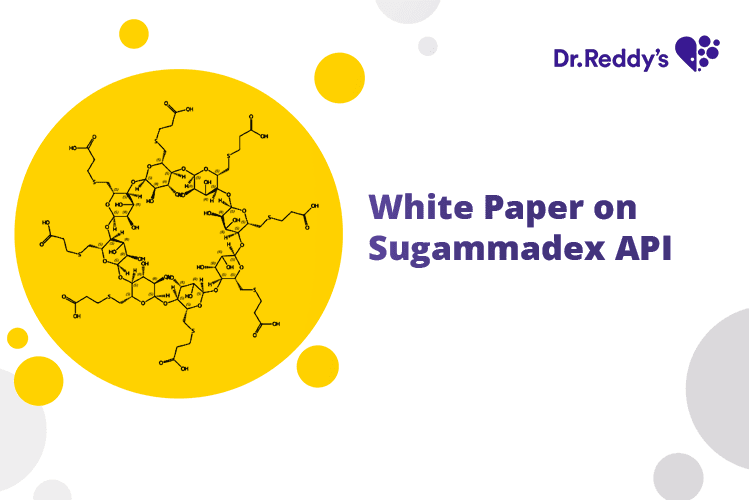
Jul 21, 2023
White Paper on Sugammadex API
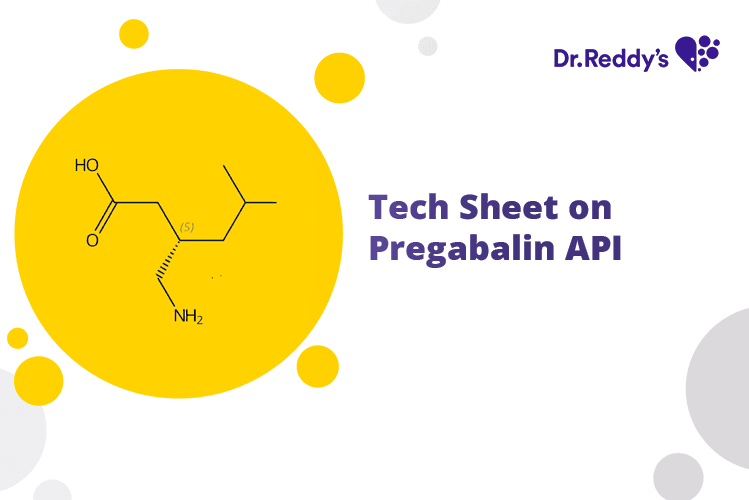
Jul 21, 2023
Tech Sheet on Pregabalin API
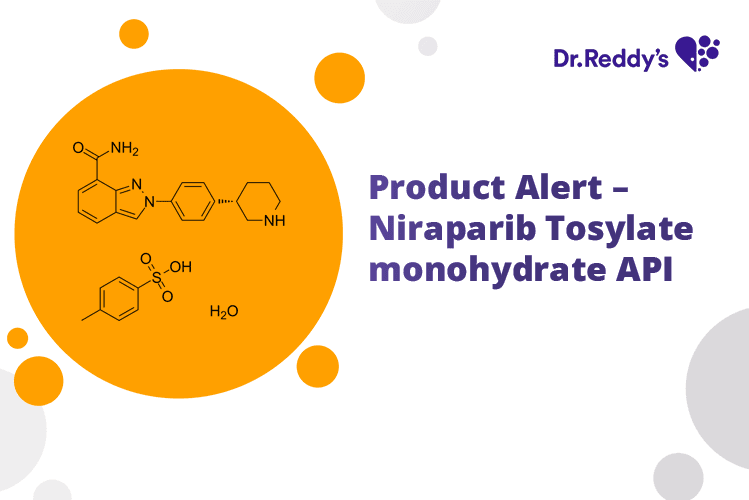
Mar 20, 2023
Product Alert – Niraparib API
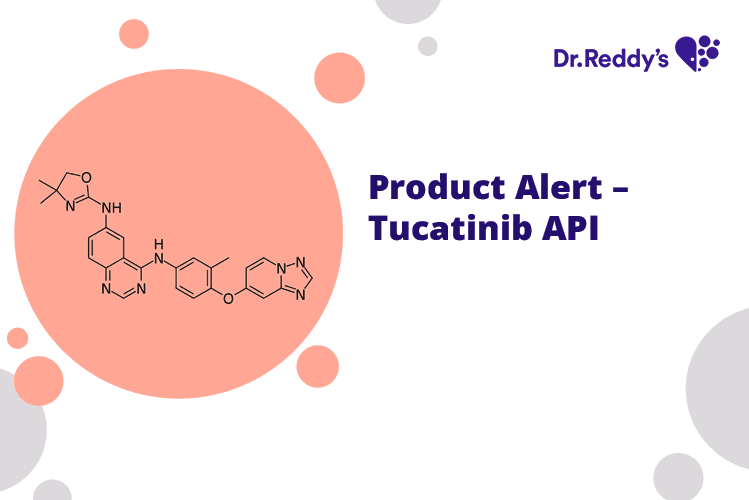
Mar 13, 2023
Product Alert – Tucatinib API
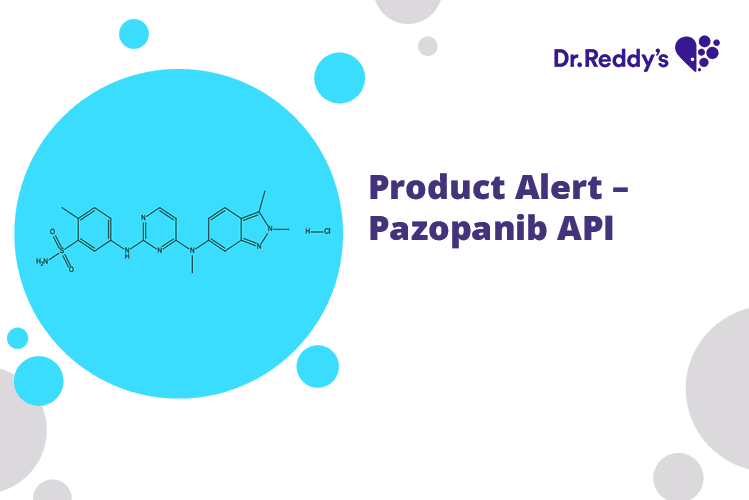
Mar 6, 2023
Product Alert – Pazopanib API
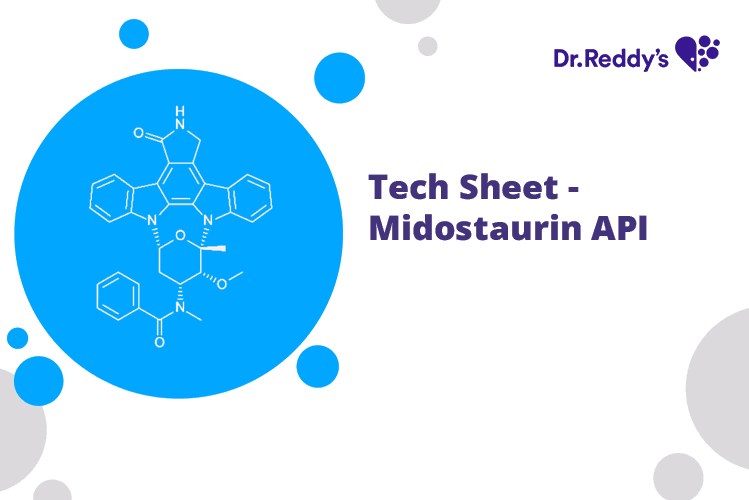
Jan 20, 2023
Tech Sheet on Midostaurin API
Jan 9, 2023
Tech Sheet on Tofacitinib Citrate
Nov 4, 2022
Tech Sheet on Dutasteride
Oct 14, 2022
Tech Sheet on Linagliptin
Oct 12, 2022
Tech Sheet on Voriconazole
Aug 3, 2022
Co-Crystal and Customized Particle Size for Early Launch Opportunity of Siponimod API
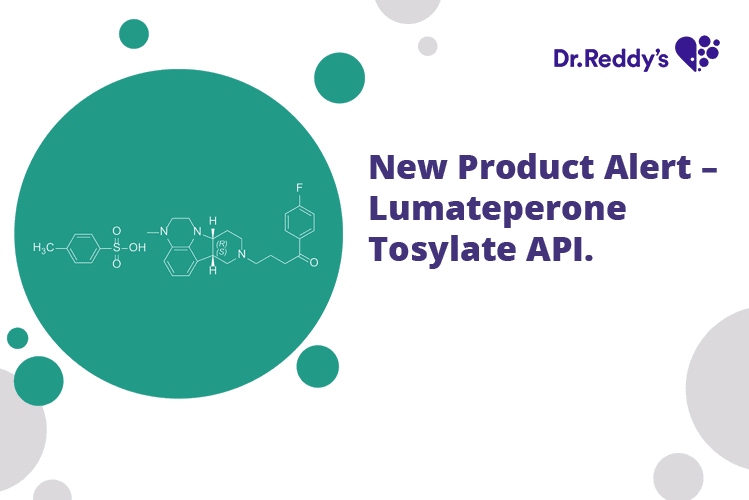
Jul 29, 2022
New Product Alert – Lumateperone Tosylate API

Jun 15, 2022
Tech sheet: Dr. Reddy's Bempedoic Acid API Offerings
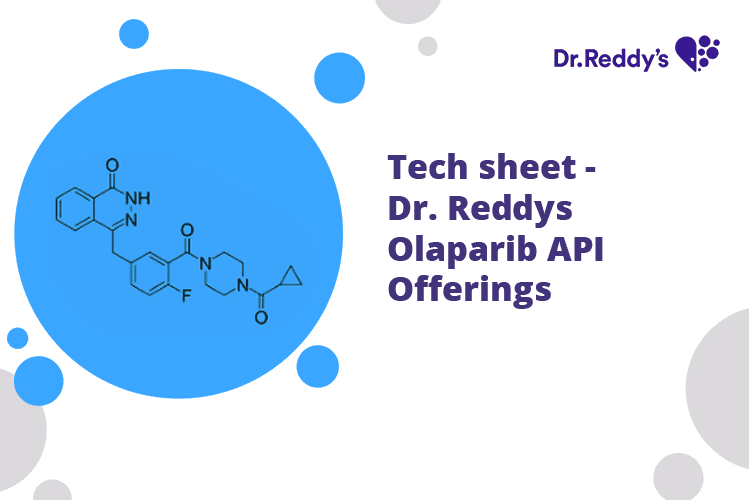
Apr 4, 2022
Tech sheet: Dr. Reddy's Olaparib API Offerings
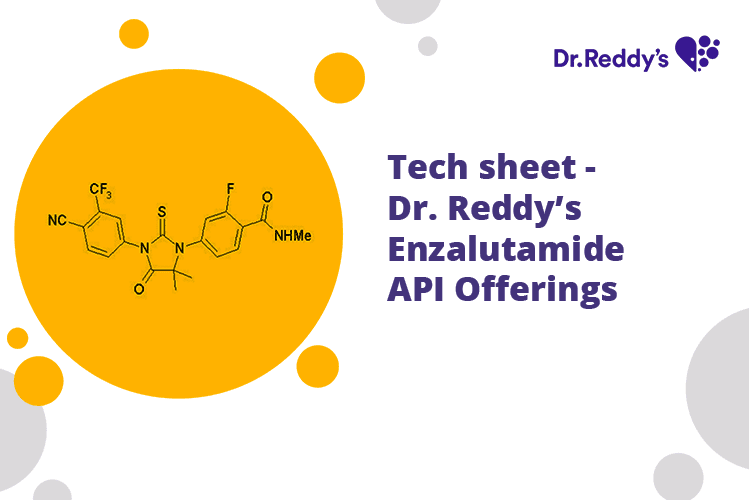
Mar 30, 2022
Tech sheet: Dr. Reddy's Enzalutamide API Offerings
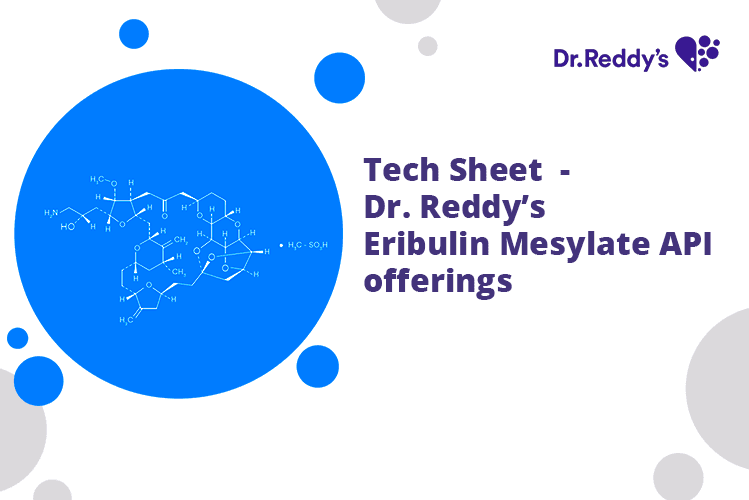
Feb 22, 2022
Tech Sheet - Dr. Reddy’s Eribulin Mesylate API offerings
Feb 9, 2022
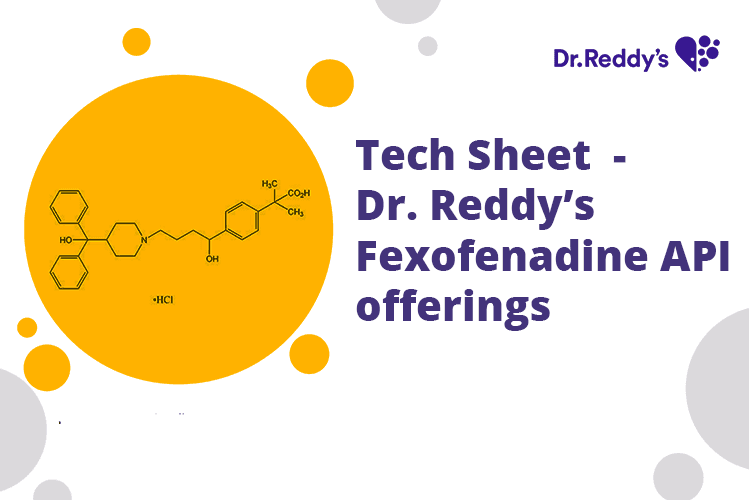
Tech Sheet – Dr. Reddy’s Fexofenadine API offerings
Feb 4, 2022
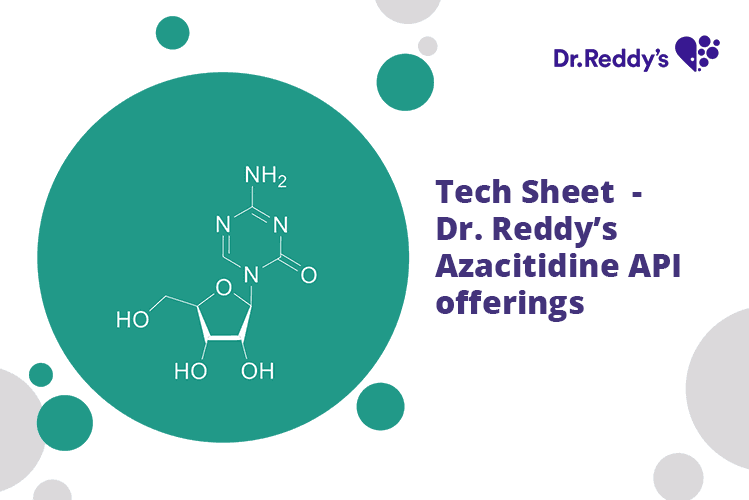
Tech Sheet - Dr. Reddy’s Azacitidine API offerings
Feb 4, 2022
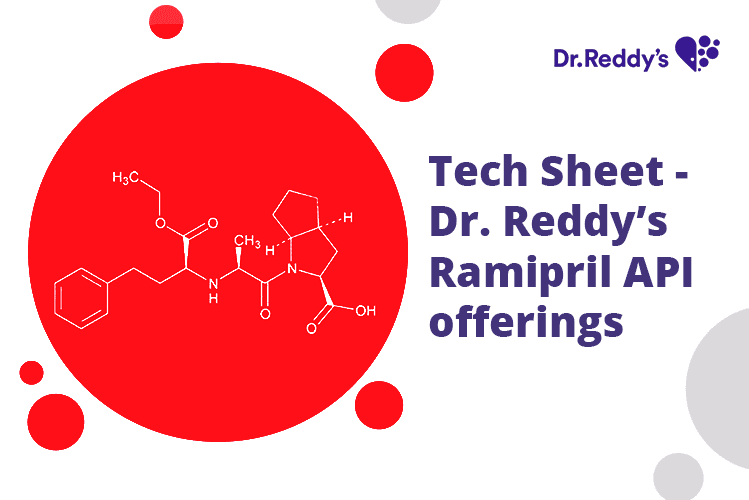
Tech Sheet - Dr. Reddy’s Ramipril API offerings
Dec 17, 2021
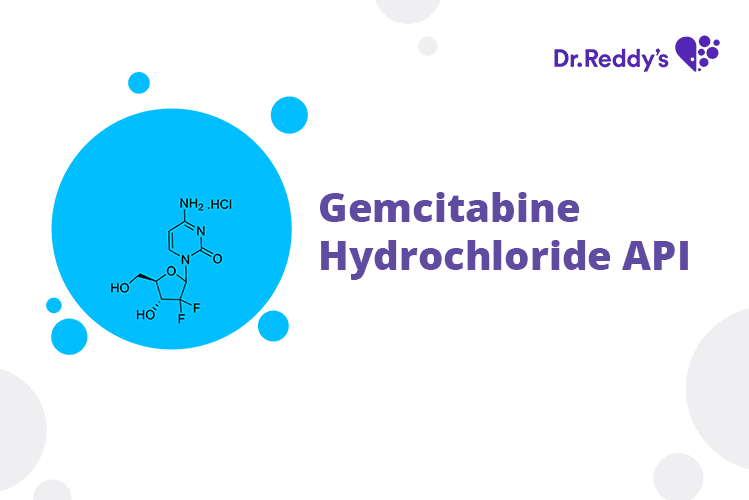
Tech Sheet - Dr. Reddy’s Gemcitabine API offerings
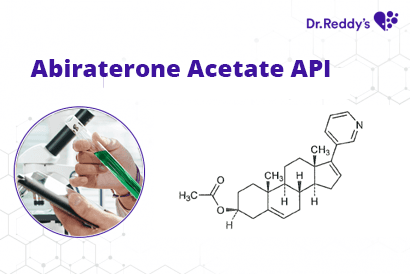
Dec 2, 2021
Tech Sheet: Dr. Reddy’s Abiraterone Acetate API offerings
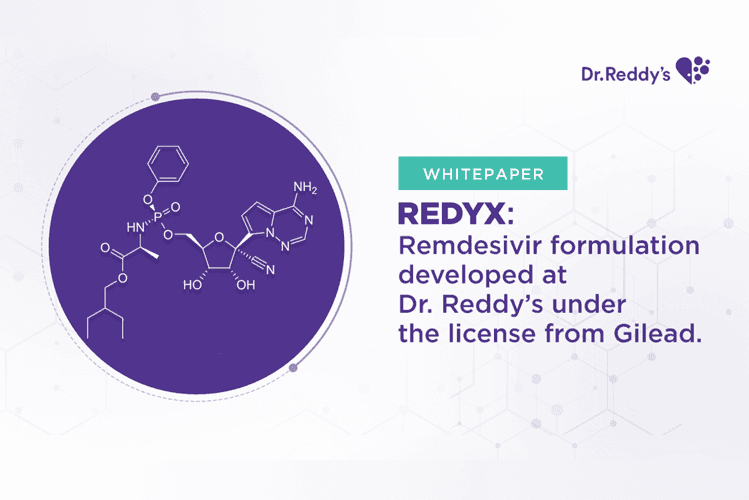
Oct 8, 2021
White Paper: Redyx: Remdesivir formulation developed at Dr. Reddy’s under the license from Gilead

Aug 17, 2021
Whitepaper : Dr.Reddy's 2 DG- A promising treatment in hospitalized COVID-19 patients
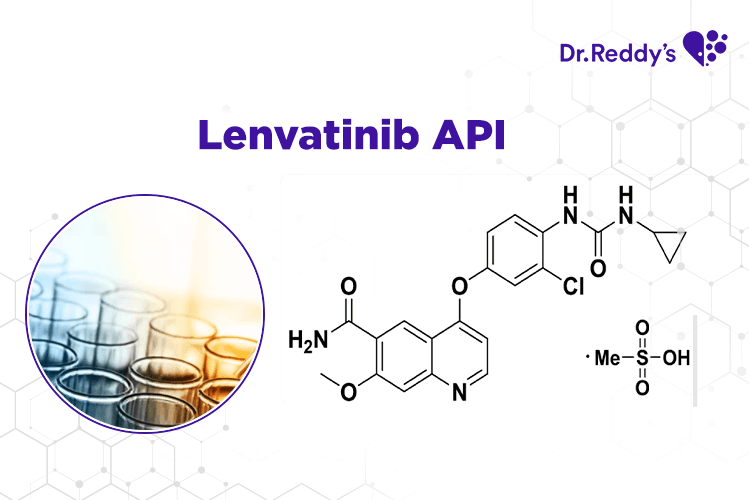
Aug 17, 2021
Techsheet: Dr.Reddy's Lenvatinib API offerings
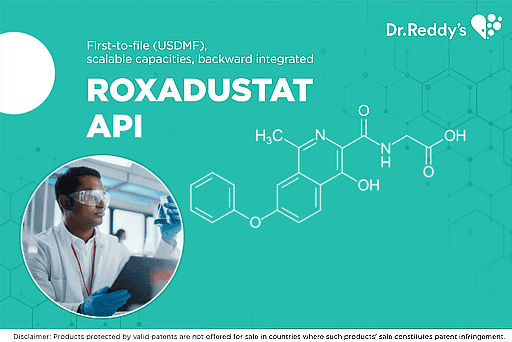
Jun 18, 2021
ROXADUSTAT - a multipronged development strategy
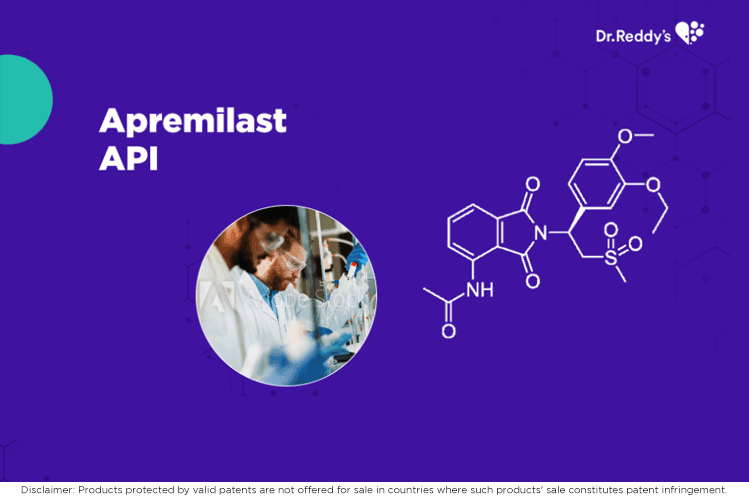
May 19, 2021
Dr.Reddy's Apremilast API and finished formulation offerings.
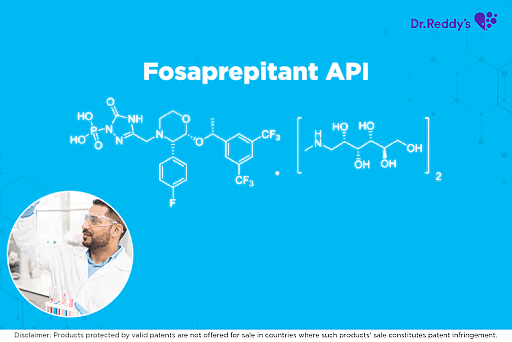
May 3, 2021
Dr. Reddy’s Fosaprepitant API offerings

Apr 15, 2021
Apixaban API – ready-to-compress granules and finished dosage forms
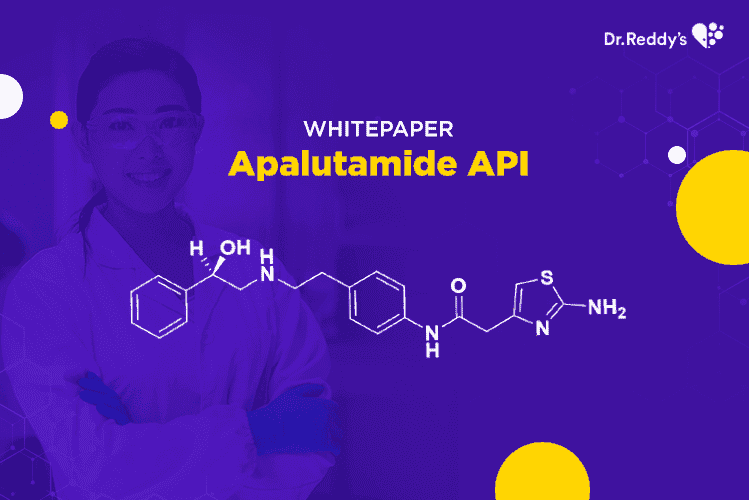
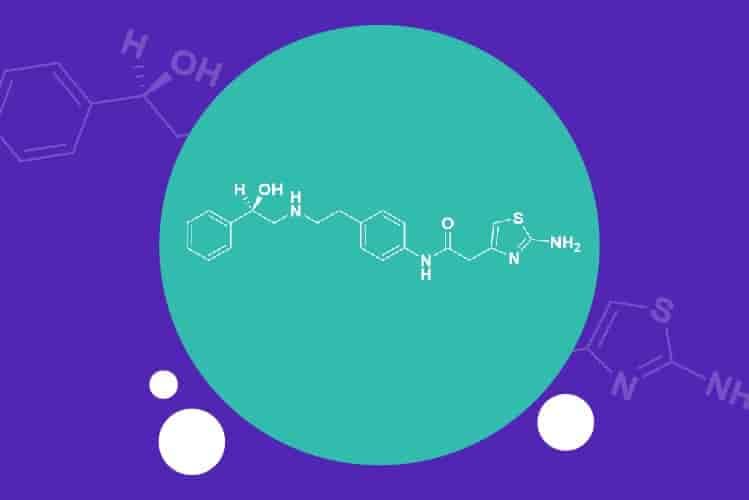
Apr 6, 2021
Apalutamide API – an attractive NCE-1 opportunity for generic pharma companies

Apr 6, 2021
Colloidal Iron Complex Formulations – Understanding and Developing the Process

Feb 9, 2021
Rivaroxaban from Dr.Reddy’s: Full basket offerings of API and Finished formulations
Jan 22, 2021
Dr. Reddy's Mirabegron API offering in both alpha and amorphous form

Jan 13, 2021
Palbociclib – how a novel process development approach provides IP advantages

Nov 25, 2020
Dr. Reddy's Elagolix API offering leverages a well rounded Quality by Design approach

Jul 20, 2020
Sacubitril/Valsartan (LCZ696) – Bringing yet another advantage through innovative API offerings

Jul 10, 2020
Dr. Reddy’s Flow Chemistry experts share their perspectives in panel discussion of Chemistry Today

May 20, 2020
A closer look on how Dr. Reddy's met the stringent regulatory requirement of genotoxic impurities in Ranolazine

May 7, 2020
Famotidine and Nizatidine APIs: NDMA controlled, customized particle distribution, supply chain security

Oct 17, 2019
Meeting the New Regulatory Requirements in Sartan API Production
Articles

Jul 25, 2025
Development of Niche Products at Dr. Reddys API

Apr 2, 2025
Harnessing India's New Drug Development Capabilities for Global Impact

Mar 11, 2025
The Impact of Global Regulations on the Active Pharmaceutical Ingredient Industry

Mar 3, 2025
The Importance of Supply Chain Management and Dr. Reddy’s Excellence

Feb 10, 2025
Current Landscape of Cancer Treatment in India and Dr. Reddy's Role in Enhancing Care

Jan 12, 2025
Global Development Strategy for Generic Formulations and APIs

Jan 6, 2025
APIs for Emerging Markets: Opportunities and Challenges
Dec 15, 2024
Formulation Strategies and Pharmaceutical Analysis for Anti-diabetic APIs

Dec 2, 2024
API Quality Control Technologies & Strategies: Current Advancements for Benefiting Pharma Companies

Oct 9, 2024
A year of growth & Sustainability
Aug 30, 2024
Understanding Nitrosamines and GTI Issues in APIs: A Comprehensive Overview with Dr. Reddy's Perspective

Aug 28, 2024
Innovative Approaches to API Production in 2024: Shaping the Future of Pharma Companies

Jul 30, 2024
Continuous Manufacturing Process and Its Impact on Pharma Manufacturing

Jun 28, 2024
A Comparative Study on Naproxen Formulations

Jun 18, 2024
An effective strategy for the development of docetaxel
May 22, 2024
Preformulation Studies for Generic Omeprazole

May 15, 2024
A comparative study on enzalutamide Formulations

Apr 30, 2024
Navigating the Heart of Pharmaceuticals: An In-Depth Exploration of Active Pharmaceutical Ingredient Manufacturing for Essential Medicines
Apr 1, 2024
Presence of Organic Impurities in active pharmaceutical ingredients: An Overview

Mar 30, 2024
Strategies and Technologies for Enhancing Cost-Effectiveness in Active Pharmaceutical Ingredient (API) Production

Mar 29, 2024
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients (APIs)

Feb 29, 2024
Ensuring Drug Safety: Comprehensive Analysis of Active Pharmaceutical Ingredient Lists
Feb 28, 2024
Unravelling the Science: Pharmaceutical Compositions and Process of Levetiracetam API

Nov 16, 2023
Impact of Venetoclax Exposure on Clinical Efficacy and Safety in Patients with Refractory Chronic Lymphocytic Leukemia

Aug 10, 2023
Demonstrating Equivalence of Generic Glatiramer Acetate: Ensuring Quality and Safety
May 25, 2023
Streamlining the Process: Preparing a Pharmaceutical Composition of Fosaprepitant API
Dec 30, 2022
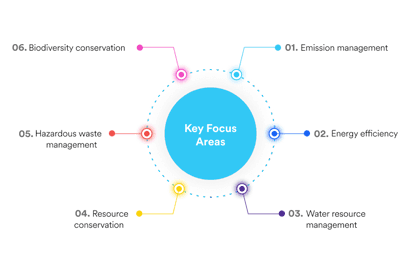
Steps taken by Dr. Reddy's Laboratories in controlling the environmental risks and safety management in the manufacturing of APIs

Dec 7, 2022
Meeting the New Regulatory Requirements and Controlling of Nitrosamine Impurities in Sartan API Production

Sep 14, 2022
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients
Sep 13, 2022
Reshaping the Pharmaceutical API Industry with Digital Transformation

Aug 2, 2022
7 Ways how Active Pharmaceutical Ingredients (APIs) Enable Indian Pharmaceutical Companies to Grow

Aug 2, 2022
How is the Indian Pharmaceutical industry gearing up for 2022-2023?
Apr 14, 2022
Impact of COVID-19 on Indian HPAPI industry
Apr 14, 2022
The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry
Sep 14, 2021
ANVISA Certification of GMP Pharmaceutical Ingredients for 61 of Dr Reddy’s APIs
Jul 7, 2021
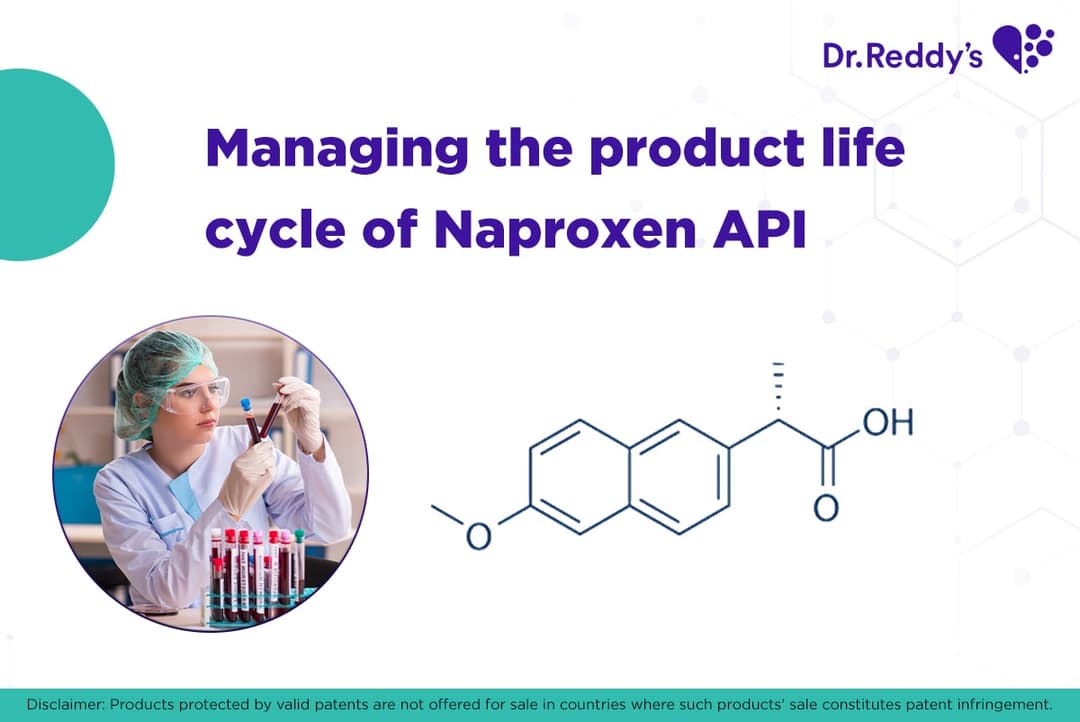
Managing the Product Life Cycle: Naproxen API and formulations

May 27, 2021
Understanding Active Pharmaceutical Ingredients: CPHI talk by Dr. Ramani Susarla
Mar 31, 2021
EMA Nitrosamine Guidance – Deadline March 31, 2021

Nov 26, 2020
Patient centricity and supply chain security in 2021 - Rajesh Sadanandan speaks on CPhI Pharma Trends 2021

Nov 19, 2020
Dr. Reddy's research publication on simultaneous automated image analysis and Raman spectroscopy of powders at an individual particle level

Sep 14, 2020
A Paradigm Shift that Is Here to Stay - Sauri Gudlavalleti speaks to CME Manager on COVID-19 response

Sep 7, 2020
A Year of Challenge and Opportunity - Deepak Sapra, Global Head of PSAI speaks to The Medicine Maker

Sep 2, 2020
COVID-19 and What’s Next for API Sourcing?

Aug 25, 2020
Indian healthcare reform offers thriving pharmaceutical market opportunities: Interview with Deepak Sapra

Jun 1, 2020
Dr. Reddy’s is improving supply chain performance for Pregabalin API through large manufacturing capacity and backward integration

Dec 1, 2019
Enhancing Supply Chain Security for Levetiracetam API
News

Oct 28, 2025
Dr. Reddys API team at CPHI Frankfurt 2025

Oct 10, 2025
A Bold Leap for Global HIV Prevention: Lenacapavir Agreement Signed

Aug 5, 2025
Tentative Approval for Generic Lumateperone capsules
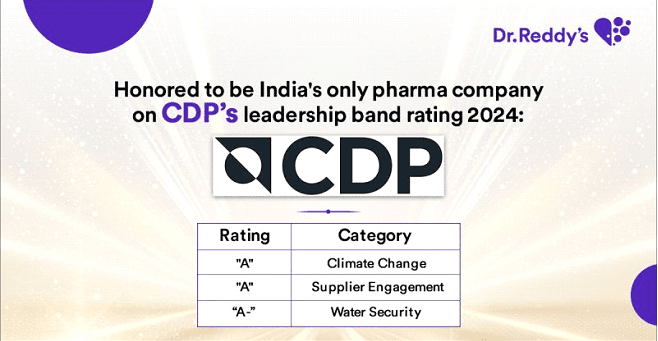
Jul 10, 2025
CDP Recognition

Jun 24, 2025
Dr. Reddys API team at CPHI China 2025

Apr 9, 2025
CPHI Japan 2025 - Exhibitor showcase session by Nirav K Shah, Vice President, Head API, North Asia

Apr 9, 2025
Dr. Reddys API team at CPHI Japan 2025

Mar 17, 2025
Dr. Reddys API team at DCAT 2025

Feb 25, 2025
Dr. Reddy’s Wins Big at “Express Pharma Excellence Awards 2025”

Dec 18, 2024
Celebrating Creativity and Festivity: API Calendar Launch 2025!

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for Excellence in Ancillary Pharma Services - Supply Chain & Logistics for enhancing customer experience

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for developing a continuous API manufacturing process.

Nov 29, 2024
Dr. Reddy's API team at CPHI India 2024

Oct 13, 2024
Dr. Reddy's API team at CPHI Milan 2024

Sep 27, 2024
Gunjan Singh at the CPHI Webinar: The Changing Dynamics of Global API Manufacturing

Sep 27, 2024
Karun Gaur at CPHI Milan: Panel Discussion on Pharma Manufacturing in Emerging Markets - Moving Towards Localization in Africa

Jul 8, 2024
Deepak Sapra's Visionary Keynote on Healthcare Innovation at DXEM 2024

May 8, 2024
Dr. Reddy's AI Day: Igniting the Future of Healthcare with Innovation

Apr 25, 2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

Apr 22, 2024
Dr. Reddy’s API at CPHI Japan 2024

Apr 5, 2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

Jan 2, 2024
Dr. Reddy's API Calendar 2024 Unveiling: A Magical Celebration of Creativity, Joy, and Togetherness with Our Global Team's Talented Young Artists

Dec 5, 2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

Nov 30, 2023
Dr. Reddy’s at CPHI India 2023

Nov 23, 2023
Dr. Reddy’s at 5th CII LIFE SCIENCES SUMMIT 2023 - Making India a Global Pharma and Lifesciences Innovation Hub

Nov 11, 2023
Asia-Pacific Climate Leader Award

Nov 10, 2023
Dr. Reddy’s Achieves AEO-T3 Certification, Elevating Global Trade Facilitation

Oct 28, 2023
Dr. Reddy’s API at CPHI Worldwide 2023

Aug 20, 2023
Dr. Reddys at G20 Summit 2023

Jun 25, 2023
Dr. Reddy’s API at CPHI China 2023

Mar 15, 2023
Being Prepared for What's Next and a Shared Purpose of Serving Patients Whatever it Takes

Feb 15, 2023
Dr. Reddy’s included in Bloomberg Gender-Equality Index for the 6th year in a row and S&P Global’s Sustainability Yearbook for the 3rd year

Jan 5, 2023
Dr. Reddy's API calendar 2023

Nov 11, 2022
COVID-19: Prime Minister of India Meets Pharma leaders

Nov 6, 2022
Addressing NDMAs in Pharmaceuticals

Nov 5, 2022
Dr.Reddy's at CPHI India 2022 Panel Discussion

Nov 4, 2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

Oct 6, 2022
Dr. Reddy's API participated in the 2022 Pharmaceutical Industry and Regulators Symposium at ANVISA

Sep 27, 2022
Expert statement by Srividya Ramakrishnan, Head, API process engineering, Dr. Reddy’s Laboratories. Published in CHE Manager

Sep 25, 2022
Dr. Reddys attended ACHEMA 2022 Conference

Nov 11, 2021
Dr. Reddy’s API team is a 2021 winner in – API Supplier of the year at Global generics & Biosimilars awards

Nov 11, 2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

Oct 7, 2021
The winner of the Best Indian Company in the US - Manufacturing Sector for the year 2021
Oct 6, 2021
Sugammadex Sodium API – CADIFA Approval by ANVISA

May 27, 2021
Deepak Sapra speaks about DRDO’s anti-covid drug 2DG

May 27, 2021
Dr.Reddy’s gearing up with Covid portfolio: Deepak Sapra, CEO of API & Services, speaks to India Today

Jan 21, 2021
Best Supply Chain Team of the Year - Pharma- 2021

Nov 10, 2020
Dr. Reddy’s Laboratories’ Anil Patni presented on HPAPIs at Manufacturing Chemist Live Virtual

Nov 3, 2020
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2020

Oct 19, 2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’

Oct 13, 2020
CPhI Festival of Pharma Roundtable: Tackling Nitrosamine Contamination

Oct 5, 2020
Dr. Reddy’s Laboratories joins Science Based Targets initiative (SBTi) and sets 2030 GHG emission targets

Oct 5, 2020
CPhI Festival of Pharma Panel discussion: Innovation Across Borders: How Pharma and innovators are fighting Back Against COVID-19 and ways forward.

Jul 1, 2020
Dr. Reddy’s partners with FUJIFILM and Global Response Aid for Avigan® (favipiravir), a potential treatment of COVID-19

Apr 14, 2020
Dr. Reddy’s statement on the COVID-19 pandemic

Apr 1, 2020
Dr. Reddy’s is featured in an article of Global Pharma Insights on “Nitrosamine contamination: pharma’s re-evaluation of its supply chain”

Apr 1, 2020
Certificate of Suitability (CEP) granted for Levofloxacin Hemihydrate

Mar 1, 2020
US DMF for Apalutamide - Now available

Mar 1, 2020
Dr. Reddy's Sustainability Report 2018-19

Dec 1, 2019
Dr. Reddy's API Team moves into its new office in China

Nov 1, 2019
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2019
Webinars
Updates
Disclaimer
No information on this website, including any reference to any product or service constitutes an offer for sale or be construed as representing an offer for sale. Products protected under valid patents are not offered or supplied for commercial use. However, in certain cases, at Dr. Reddy's sole discretion, and subject to local legal requirement, the research quantities of such products may be offered for the purpose of regulatory submissions under Section 107A of the Indian Patent Act (Bolar exemption), wherever such regulatory exemptions exist. The buyers should make their independent evaluation of the product or service including, patent scenario in their respective markets and will be responsible for all patent related liabilities Dr. Reddy's disclaims all warranties, express or implied, including but not limited to warranties of merchantability, fitness for a particular purpose and non-infringement.
